Difference between revisions of "Acetone"
| (41 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
| + | Solvent for easy cleaning. A necessity for working with Resin. | ||
| − | + | ==Overview== | |
| − | |||
Acetone is a useful solvent for the plastics industry. Furthermore it has many general purpose uses, and is quite safe to humans in small amounts - it is not dangerous unless exposed to fire or ingested. It can be purchased at the grocery store as Nail Polish remover. Small amounts of Acetone evaporate when exposed to air. | Acetone is a useful solvent for the plastics industry. Furthermore it has many general purpose uses, and is quite safe to humans in small amounts - it is not dangerous unless exposed to fire or ingested. It can be purchased at the grocery store as Nail Polish remover. Small amounts of Acetone evaporate when exposed to air. | ||
==Safety== | ==Safety== | ||
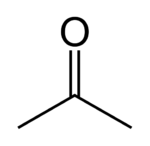
| + | [[File:492px-Acetone-2D-skeletal.svg.png|150px|thumb|right|Acetone is the basic ketone]] | ||
Acetone is flammable. Store Acetone in a closed container. Dirty Acetone can be saved and re-used until you deem it is too dirty. It is not corrosive in small amounts, and does little more than dry human skin (though keep away from open wounds). For full details of the health and safety of Acetone refer to a MSDS/SDS. | Acetone is flammable. Store Acetone in a closed container. Dirty Acetone can be saved and re-used until you deem it is too dirty. It is not corrosive in small amounts, and does little more than dry human skin (though keep away from open wounds). For full details of the health and safety of Acetone refer to a MSDS/SDS. | ||
| + | |||
| + | Use in a well ventilated area. More or less, the only way you will get in trouble is either being near a source of flame, or working in a small room without ventilation (and using a lot of acetone). | ||
| + | |||
| + | If you do manage to get acetone on fire, it will burn like an alcohol. But it will not explode. Acetone vapours however are flammable, and can cause an airborne flame. If you somehow manage to boil the acetone, this can be a danger (a spark can cause acetone gas to ignite in a fireball). Understand the potential of what you are working with. | ||
| + | |||
| + | ==Fire Hazard== | ||
| + | {{Template:FlammableRedLabel}} | ||
==How to Use Acetone== | ==How to Use Acetone== | ||
===Cleaning Tools=== | ===Cleaning Tools=== | ||
| − | Acetone can be used to clean off many kinds of uncured and cured plastics and polymers from tools. Tools can be soaked in an acetone bath over time. It | + | Acetone can be used to clean off many kinds of uncured and cured plastics and polymers from tools. Tools can be soaked in an acetone bath over time. It can be used along with e.g. a metal drywall scraper to remove slightly cured resin from surfaces. Be aware that some plastics will be damaged by acetone, so test in an inconspicuous spot when uncertain. |
===Preparing Surfaces=== | ===Preparing Surfaces=== | ||
| − | In cleaning a concrete floor | + | In cleaning a concrete floor, Acetone can be used as an alternative to water as it will evaporate quicker. This is done when preparing a concrete floor for finishing (epoxy, urethane, etc). It also helps to clean any detergents or oils left on e.g. epoxy floors after they are have been cured. |
===Cleaning Hands=== | ===Cleaning Hands=== | ||
| − | Acetone can be used to clean resins off of hands or surfaces. | + | Acetone can be used to clean resins off of hands or surfaces. Apply to a rag, and wipe your hands in the rag, until the resin is dissolved in the solvent. |
| + | |||
| + | ==Tips / Techniques== | ||
| + | ===Storage=== | ||
| + | Acetone is generally best stored in a metal container, such as our 1 gallon cans. Glass is another good option. It can be stored in 'some' types of plastic containers, but others will degrade if used to store acetone, so you need to have the right kind of plastic. There are charts online to refer to, if you must store it in plastic. (reference: http://web.archive.org/web/20240122003118/https://cdn7.bigcommerce.com/s-3yvzqa/content/v/images/custom/Chemical_Reference_Summary-small.gif - In this chart, Acetone is considered a 'ketone' - it's the fundamental ketone) | ||
| + | |||
| + | ===Retail Store Acetone=== | ||
| + | Acetone can be purchased from grocery stores in household amounts. It can also be purchased from Advance Coatings in small to large quantities. Note that these acetones are not 100% equal. The quality from consumer/retail stores tends to have a more recycled smell/scent, than what we sell. As someone who has used Advance Coatings acetone for many years, I instantly recognize that consumer acetone has a 'dirtier' or 'rougher' scent to it. It's not as pure. Not including nail polish remover, which is only some percentage of acetone, along with fragrance. But even consumer supposedly 100% pure acetone can vary in quality. | ||
| + | |||
| + | For general purposes it may not matter, but it is something to be aware of. | ||
| + | |||
| + | ===Reusing Acetone=== | ||
| + | We reuse acetone as much as possible. Even when Acetone gets dirty, it is still a viable solvent. It's wise to store different levels of 'dirty' acetone. I.e. keep a container of pure acetone for finish cleaning. And keep a container or two of dirtier acetone for rough cleaning. | ||
| + | |||
| + | ===Acetone Exposed to Air will Evaporate=== | ||
| + | Keep acetone containers closed when not in use. It will evaporate into the air (and is flammable). This can also be of benefit, i.e. if you pour some acetone onto a surface for cleaning, it will evaporate relatively quickly. | ||
| + | |||
| + | ==External Links== | ||
| + | * https://en.wikipedia.org/wiki/Acetone | ||
{{Tools}} | {{Tools}} | ||
| + | [[Category:Products]] | ||
| + | [[Category:ResinAdditives]] | ||
Latest revision as of 02:09, 1 November 2024
Solvent for easy cleaning. A necessity for working with Resin.
Overview
Acetone is a useful solvent for the plastics industry. Furthermore it has many general purpose uses, and is quite safe to humans in small amounts - it is not dangerous unless exposed to fire or ingested. It can be purchased at the grocery store as Nail Polish remover. Small amounts of Acetone evaporate when exposed to air.
Safety
Acetone is flammable. Store Acetone in a closed container. Dirty Acetone can be saved and re-used until you deem it is too dirty. It is not corrosive in small amounts, and does little more than dry human skin (though keep away from open wounds). For full details of the health and safety of Acetone refer to a MSDS/SDS.
Use in a well ventilated area. More or less, the only way you will get in trouble is either being near a source of flame, or working in a small room without ventilation (and using a lot of acetone).
If you do manage to get acetone on fire, it will burn like an alcohol. But it will not explode. Acetone vapours however are flammable, and can cause an airborne flame. If you somehow manage to boil the acetone, this can be a danger (a spark can cause acetone gas to ignite in a fireball). Understand the potential of what you are working with.
Fire Hazard
Warning: This product is Flammable.
How to Use Acetone
Cleaning Tools
Acetone can be used to clean off many kinds of uncured and cured plastics and polymers from tools. Tools can be soaked in an acetone bath over time. It can be used along with e.g. a metal drywall scraper to remove slightly cured resin from surfaces. Be aware that some plastics will be damaged by acetone, so test in an inconspicuous spot when uncertain.
Preparing Surfaces
In cleaning a concrete floor, Acetone can be used as an alternative to water as it will evaporate quicker. This is done when preparing a concrete floor for finishing (epoxy, urethane, etc). It also helps to clean any detergents or oils left on e.g. epoxy floors after they are have been cured.
Cleaning Hands
Acetone can be used to clean resins off of hands or surfaces. Apply to a rag, and wipe your hands in the rag, until the resin is dissolved in the solvent.
Tips / Techniques
Storage
Acetone is generally best stored in a metal container, such as our 1 gallon cans. Glass is another good option. It can be stored in 'some' types of plastic containers, but others will degrade if used to store acetone, so you need to have the right kind of plastic. There are charts online to refer to, if you must store it in plastic. (reference: http://web.archive.org/web/20240122003118/https://cdn7.bigcommerce.com/s-3yvzqa/content/v/images/custom/Chemical_Reference_Summary-small.gif - In this chart, Acetone is considered a 'ketone' - it's the fundamental ketone)
Retail Store Acetone
Acetone can be purchased from grocery stores in household amounts. It can also be purchased from Advance Coatings in small to large quantities. Note that these acetones are not 100% equal. The quality from consumer/retail stores tends to have a more recycled smell/scent, than what we sell. As someone who has used Advance Coatings acetone for many years, I instantly recognize that consumer acetone has a 'dirtier' or 'rougher' scent to it. It's not as pure. Not including nail polish remover, which is only some percentage of acetone, along with fragrance. But even consumer supposedly 100% pure acetone can vary in quality.
For general purposes it may not matter, but it is something to be aware of.
Reusing Acetone
We reuse acetone as much as possible. Even when Acetone gets dirty, it is still a viable solvent. It's wise to store different levels of 'dirty' acetone. I.e. keep a container of pure acetone for finish cleaning. And keep a container or two of dirtier acetone for rough cleaning.
Acetone Exposed to Air will Evaporate
Keep acetone containers closed when not in use. It will evaporate into the air (and is flammable). This can also be of benefit, i.e. if you pour some acetone onto a surface for cleaning, it will evaporate relatively quickly.
External Links
| ||||||||||||||